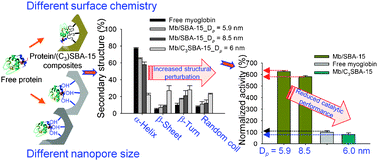
The interactions of proteins with the surface of cylindrical nanopores are systematically investigated to elucidate how surface curvature and surface chemistry affect the conformation and activity of confined proteins in an aqueous, buffered environment. Two globular proteins, lysozyme and myoglobin, with different catalytic functions, were used as model proteins to analyze structural changes in proteins after adsorption on ordered mesoporous silica SBA-15 and propyl-functionalized SBA-15 (C3SBA-15) with carefully controlled pore size. Liquid phase ATR-FTIR spectroscopy was used to study the amide I and II bands of the adsorbed proteins. The amide I bands showed that the secondary structures of free and adsorbed protein molecules differ, and that the secondary structure of the adsorbed protein is influenced by the local geometry as well as by the surface chemistry of the nanopores. The conformation of the adsorbed proteins inside the nanopores of SBA-15 and C3SBA-15 is strongly correlated with the local geometry and the surface properties of the nanoporous materials, which results in different catalytic activities. Adsorption by electrostatic interaction of proteins in nanopores of an optimal size provides a favorably confining and protecting environment, which may lead to considerably enhanced structural stability and catalytic activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...