Mechanistic differences between methanol and dimethyl ethercarbonylation in side pockets and large channels of mordenite
Abstract
The activity and selectivity towards
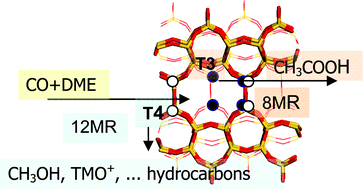
- This article is part of the themed collection: Controlled nanostructures for applications in catalysis

 Please wait while we load your content...
Please wait while we load your content...