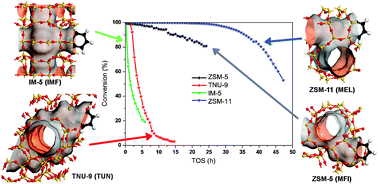
Four 3D 10-ring zeolites, IM-5, TNU-9, ZSM-11 and ZSM-5, with Si/Al = 14–24 and crystal sizes below 2 microns, were tested as catalysts for the methanol to hydrocarbons reaction (MTH) at atmospheric pressure, 350 °C and WHSV = 9 h−1. All catalysts gave initially full methanol conversion, and showed strikingly similar effluent product selectivities. However, their life-time duration differed significantly, and decreased in the order: ZSM-11 > ZSM-5 ≫ TNU-9 > IM-5. A main difference between the two groups of stability behaviour was the size of cavities formed by channel intersections; larger cavities in TNU-9 and IM-5 leading to polyaromatics formation and a more rapid deactivation compared to ZSM-5 and ZSM-11. Effluent yield–conversion plots suggested that polymethylated benzene intermediates were more important in IM-5 and TNU-9 than in ZSM-5 and ZSM-11, where alkene methylation and cracking reactions seemed to dominate product formation. However, this difference had only minor influence on effluent selectivity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...