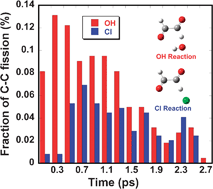
On-the-fly quasi-classical trajectory calculations using the density functional method were carried out to investigate the dynamics of the HC(O)CO radical, formed by OH radical- and Cl atom-initiated reactions of glyoxal at 298 K. The energy difference between the A′ HC(O)CO radical, formed immediately after H atom abstraction, and the most stable A″ HC(O)CO radical is estimated to be 6.0 kcal mol−1. The surplus energy followed by relaxation from A′ HC(O)CO to A″ HC(O)CO goes to internal energy of the nascent HC(O)CO radicals and causes prompt decomposition into HCO + CO. The average internal energy partitioned into the HC(O)CO radical is higher in the OH reaction than in the Cl reaction, in accordance with exothermicity of the reactions. A fraction of the nascent HC(O)CO radicals (91% for the OH reaction and 47% for the Cl reaction) promptly decomposes into HCO and CO within 2.5 ps. The remaining HC(O)CO radicals, which do not undergo prompt decomposition, decompose thermally or add with O2 in the presence of O2. I re-evaluated the previous two experiment results of the product yield ratio [CO]/[CO2] vs. [O2]−1 in the Cl atom-initiated reaction, in light of the reaction mechanism involving prompt decomposition. The two results give 9.5 × 106 s−1 and 1.08 × 107 s−1 for the thermal decomposition rate and 47% and 41% for the fraction of prompt decomposition in the Cl atom-initiated reaction, in good agreement with the present trajectory calculation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...