Relativistic DFT calculations of the NMR properties and reactivity of transition metal methane σ-complexes: insights on C–H bond activation†
Abstract
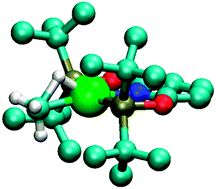
Relativistic ZORA DFT methods have been employed to predict the NMR properties of methane and methyl hydride complexes of rhodium and iridium. Two of these compounds, the rhodium methane and the iridium methyl hydride complexes, have been recently characterized by

 Please wait while we load your content...
Please wait while we load your content...