Thermodynamic calculation and interatomic potential to predict the favored composition region for the Cu–Zr–Al metallic glass formation
Abstract
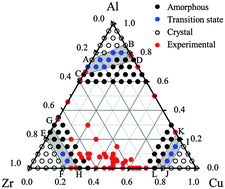
For the Cu–Zr–Al system, the glass forming compositions were firstly calculated based on the extended Miedema's model, suggesting that the amorphous phase could be thermodynamically favored over a large composition region. An n-body potential was then constructed under the smoothed and long-range second-moment-approximation of tight-binding formulism. Applying the constructed Cu–Zr–Al potential, molecular dynamics simulations were conducted using solid solution models to compare relative stability of crystalline solid solution versus its disordered counterpart. Simulations reveal that the physical origin of metallic glass formation is crystalline lattice collapsing while solute concentration exceeding the critical value, thus predicting a hexagonal composition region, within which the Cu–Zr–Al ternary metallic glass formation is energetically favored. The molecular dynamics simulations predicted composition region is defined as the quantitative glass-forming-ability or glass-forming-region of the Cu–Zr–Al system.

 Please wait while we load your content...
Please wait while we load your content...