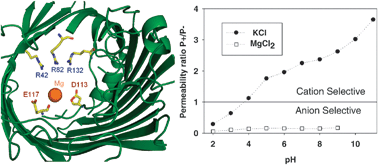
In contrast to the highly-selective channels of neurophysiology employing mostly the exclusion mechanism, different factors account for the selectivity of large channels. Elucidation of these factors is essential for understanding the permeation mechanisms in ion channels and their regulation in vivo. The interaction between divalent cations and a protein channel, the bacterial porin OmpF, has been investigated paying attention to the channel selectivity and its dependence on the solution pH. Unlike the experiments performed in salts of monovalent cations, the channel is now practically insensitive to pH, being anion selective all over the pH range considered. Electrostatic calculations based on the available structural data suggest that the binding of divalent cations has two main effects: (i) the pKa values of key ionizable groups differ significantly from those of the isolated groups in solution and (ii) the cation binding has a decisive impact on the effective electric charge regulating the channel selectivity. A simple molecular model based on statistical thermodynamics provides additional qualitative explanations to the experimental findings that could also be useful for other related systems like synthetic nanopores, ion exchange membranes, and polyelectrolyte multilayers.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...