Spectroscopic and computational study of β-ethynylphenylene substituted zinc and free-base porphyrins†
Abstract
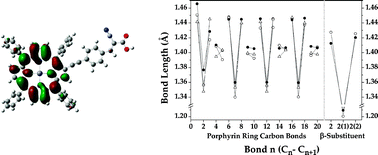
A series of tetraphenylporphyrins appended at the β-pyrrolic position with an ethynylphenylene- or ethynylpyridine-substituent have been subjected to spectroscopic and density functional theory (DFT) analyses. The mean absolute deviation between corresponding experimental and DFT-derived

 Please wait while we load your content...
Please wait while we load your content...