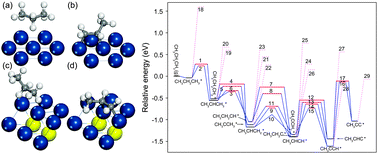
Self-consistent periodic slab calculations based on gradient-corrected density functional theory (DFT-GGA) have been conducted to examine the reaction network of propane dehydrogenation over close-packed Pt(111) and stepped Pt(211) surfaces. Selective C–H or C–C bond cleaving is investigated to gain a better understanding of the catalyst site requirements for propane dehydrogenation. The energy barriers for the dehydrogenation of propane to form propylene are calculated to be in the region of 0.65–0.75 eV and 0.25–0.35 eV on flat and stepped surfaces, respectively. Likewise, the activation of the side reactions such as the deep dehydrogenation and cracking of C3 derivatives depends strongly on the step density, arising from the much lower energy barriers on Pt(211). Taking the activation energy difference between propylene dehydrogenation and propylene desorption as the descriptor, we find that while step sites play a crucial role in the activation of propane dehydrogenation, the selectivity towards propylene is substantially lowered in the presence of the coordinatively unsaturated surface Pt atoms. As the sole C3 derivative which prefers the cleavage of the C–C bond to the C–H bond breaking, propyne is suggested to be the starting point for the C–C bond breaking which eventually gives rise to the formation of ethane, methane and coke. These findings provide a rational interpretation of the recent experimental observations that smaller Pt particles containing more step sites are much more active but less selective than larger particles in propane dehydrogenation.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...