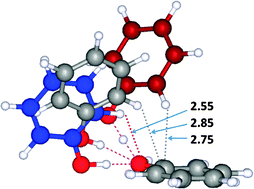
We have carried out extensive calculations for neutral, cationic protonated, anionic deprotonated phenol dimers. The structures and energetics of this system are determined by the delicate competition between H-bonding, H–π interaction and π–π interaction. Thus, the structures, binding energies and frequencies of the dimers are studied by using a variety of functionals of density functional theory (DFT) and Møller–Plesset second order perturbation theory (MP2) with medium and extended basis sets. The binding energies are compared with those of highly reliable coupled cluster theory with single, double, and perturbative triple excitations (CCSD(T)) at the complete basis set (CBS) limit. The neutral phenol dimer is unique in the sense that its experimental rotational constants have been measured. The geometry of the neutral phenol dimer is governed by the hydrogen bond formed by two hydroxyl groups and the H–π interaction between two aromatic rings, while the structure of the protonated/deprotonated phenol dimers is additionally governed by the electrostatic and induction effects due to the short strong hydrogen bond (SSHB) and the charges populated in the aromatic rings in the ionic systems. Our salient finding is the substantial differences in structure between neutral, protonated, and deprotonated phenol dimers. This is because the neutral dimer involves in both Hπ⋯O and Hπ⋯π interactions, the protonated dimer involves in Hπ⋯π interactions, and the deprotonated dimer involves in a strong Hπ⋯O interaction. It is important to compare the reliability of diverse computational approaches employed in quantum chemistry on the basis of the calculational results of this system. MP2 calculations using a small cc-pVDZ basis set give reasonable structures, but those using extended basis sets predict wrong π-stacked structures due to the overestimation of the dispersion energies of the π–π interactions. A few new DFT functionals with the empirical dispersion give reliable results consistent with the CCSD(T)/CBS results. The binding energies of the neutral, cationic protonated, and anionic deprotonated phenol dimers are estimated to be more than 28.5, 118.2, and 118.3 kJ mol−1, respectively. The energy components of the intermolecular interactions for the neutral, protonated and deprotonated dimers are analyzed.

 Please wait while we load your content...
Please wait while we load your content...