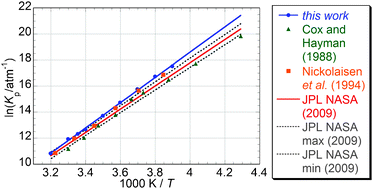
Recent work by von Hobe et al. [Atmos. Chem. Phys., 2007, 7, 3055] has highlighted significant inconsistencies between laboratory results, theoretical calculations and field observations concerning the ClO dimer ozone destruction cycle. This work investigates the temperature dependence of the equilibrium constant of one of the key reactions in this cycle, ClO + ClO + M ⇆ Cl2O2 + M (1, −1), by means of laser flash photolysis and time-resolved UV absorption spectroscopy. ClO radicals were generated via laser flash photolysis of Cl2/Cl2O mixtures in synthetic air. Radicals were monitored via UV absorption spectroscopy: the use of a charge coupled device (CCD) detector allowed time resolution over a broad range of wavelengths giving unequivocal concentrations of radicals. The equilibrium constant Keq was determined as the ratio of the rate constants of the forward and reverse reactions (1, −1) over the temperature range T = 256.55–312.65 K. Second Law and Third Law thermodynamic methods were employed to determine the standard enthalpy and entropy changes of reaction (1), ΔrH° and ΔrS°, from the measured equilibrium constants. The values obtained from Second Law analysis were ΔrH° = − 80.7 ± 2.2 kJ mol−1 and ΔrS° = −168.1 ± 7.8 J K−1 mol−1. Third Law analysis gave ΔrH° = −74.65 ± 0.4 kJ mol−1 and ΔrS° = −148.0 ± 0.4 J K−1 mol−1. These values are in good agreement with previous work by Nickolaisen et al. [J. Phys. Chem., 1994, 98, 155] but greater in (negative) magnitude than current JPL-NASA recommendations [Sander et al., Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, JPL Publication 06-2, NASA Jet Propulsion Laboratory, Pasadena, 2006 (interim update to this reference, 2009)]. The discrepancy between the Second and Third Law analyses also agrees with Nickolaisen et al., possibly indicating an aspect of the ClO recombination reaction not yet fully elucidated. The atmospheric implications of the results and their impact on the current understanding on polar ozone depletion are briefly discussed.

 Please wait while we load your content...
Please wait while we load your content...