In a situation which is far from ideal, many buffers have been found to be quite reactive, besides maintaining their stable pH values. On the basis of apparent transfer free energies (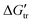 ), through solubility measurements the interactions of zwitterionic glycine peptides: glycine (Gly), diglycine (Gly2), triglycine (Gly3), and tetraglycine (Gly4), with several common neutral pH, amine-based buffers have been studied. The biological buffers studied in this work, including TRIS, TES, TAPS, TAPSO, and TABS are structurally related and all contain TRIS groups. These buffers have pKa values ranging from 7.5–9.0, which allow them to be used in biological, biochemical or environmental studies. We observed negative values of
), through solubility measurements the interactions of zwitterionic glycine peptides: glycine (Gly), diglycine (Gly2), triglycine (Gly3), and tetraglycine (Gly4), with several common neutral pH, amine-based buffers have been studied. The biological buffers studied in this work, including TRIS, TES, TAPS, TAPSO, and TABS are structurally related and all contain TRIS groups. These buffers have pKa values ranging from 7.5–9.0, which allow them to be used in biological, biochemical or environmental studies. We observed negative values of 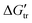 for Gly3 and Gly4 from water to buffer, indicating that the interactions are favorable. However, the
for Gly3 and Gly4 from water to buffer, indicating that the interactions are favorable. However, the 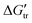 values are positive for Gly and Gly2, revealing unfavorable interactions, which except for the latter in TRIS buffer are negative. The surprising result in our data is the unexpected extraordinarily high favorable interactions between TRIS buffer and peptides (in comparison with the effect of the most common denaturants, urea and guanidine hydrochloride). The transfer free energies (
values are positive for Gly and Gly2, revealing unfavorable interactions, which except for the latter in TRIS buffer are negative. The surprising result in our data is the unexpected extraordinarily high favorable interactions between TRIS buffer and peptides (in comparison with the effect of the most common denaturants, urea and guanidine hydrochloride). The transfer free energies (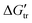 ) of the peptide backbone unit (–CH2C
) of the peptide backbone unit (–CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–NH–) contributions have been estimated from
O–NH–) contributions have been estimated from 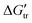 values. We have also investigated the interactions of TRIS buffer with Bovine Serum Albumin (BSA), as a globular protein, using dynamic light scattering (DLS), zeta potential, UV-Visible absorption, fluorescence and Raman spectroscopy measurements. The results indicated that TRIS buffer stabilized the BSA molecules.
values. We have also investigated the interactions of TRIS buffer with Bovine Serum Albumin (BSA), as a globular protein, using dynamic light scattering (DLS), zeta potential, UV-Visible absorption, fluorescence and Raman spectroscopy measurements. The results indicated that TRIS buffer stabilized the BSA molecules.
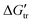 ), through solubility measurements the interactions of
), through solubility measurements the interactions of 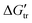 for Gly3 and
for Gly3 and 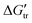 values are positive for
values are positive for 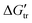 ) of the
) of the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–NH–) contributions have been estimated from
O–NH–) contributions have been estimated from 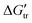 values. We have also investigated the interactions of TRIS
values. We have also investigated the interactions of TRIS ![Graphical abstract: Interactions of TRIS [tris(hydroxymethyl)aminomethane] and related buffers with peptide backbone: Thermodynamic characterization](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C0CP00253D&imageInfo.ImageIdentifier.Year=2010)

 Please wait while we load your content...
Please wait while we load your content...