Ion-size effect within the aqueous solution interface at the Pt(111) surface: molecular dynamics studies
Abstract
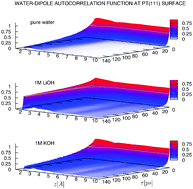
All-atom classical force-field based molecular dynamics simulations have been employed to investigate the structure and dynamics of interfacial

 Please wait while we load your content...
Please wait while we load your content...