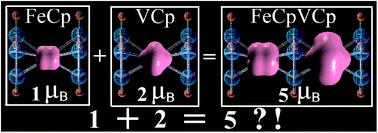
Using density functional theory calculations, we demonstrate that one-dimensional bimetallic molecular ferromagnets (FeCpMCp)∞ (M = Sc, Ti, V, Cr and Mn, Cp = cyclopentadienyl) exhibit significant enhancement in local and global atomic magnetic moments as well as relatively large spin-polarization energy as compared to their monometallic counterparts. These yield an unusual charge configuration for one of the wires: (Fe0Cp−1V+2Cp−1)∞. Hückel's rule and double-exchange interaction model are used to illustrate the details of local charge transfer and long-range ferromagnetic order. We then propose a growth mechanism for Vn(FeCp2)(n+1) (n = 1–4) clusters, which is supported unambiguously with time-of-flight mass spectroscopy data.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...