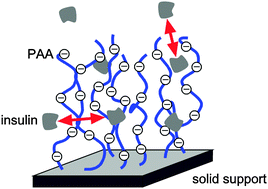
A planar poly(acrylic acid) (PAA) brush provides an unusual substrate for the unspecific immobilization of proteins on material surfaces. At neutral pH-values, proteins adsorb at a PAA brush when the ionic strength of the protein solution is low. In contrast, raising the ionic strength to a few 100 mM transforms a PAA brush into a rather protein-resistant surface coating. Moreover, a PAA brush represents a mild environment for adsorbed proteins which preserves their secondary structure and biological activity. In this study, we focus on the biocompatibility of a PAA brush with an insulin solution. Insulin can form amyloid fibrils, which may also be triggered by interfaces. Using neutron reflectometry and attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectroscopy, the effects of pD value, ionic strength, and glycerol concentration on the density profile and the secondary structure of adsorbed insulin molecules at a PAA brush have been studied. At pD 7, insulin adsorbs at a PAA brush despite its negative net charge. As has been found for other proteins in earlier studies, increasing the ionic strength of the insulin solution to 500 mM decreases the amount of adsorbed insulin drastically. In contrast, at pD 2, addition of salt to the insulin solution induces a thick insulin adsorbate at a PAA brush suggesting both protein–brush and protein–protein interactions, i.e., insulin adsorption and aggregation to be effective. However, in the presence of 2 M glycerol, insulin adsorption is largely suppressed. Furthermore, no major alterations of the secondary structure of adsorbed insulin can be detected by ATR-FTIR spectroscopy under all conditions studied. Hence, the performed experiments demonstrate that a PAA brush does not promote the formation of insulin amyloid structures, which represents a fundamentally new aspect of the biocompatibility of this material surface coating.

 Please wait while we load your content...
Please wait while we load your content...