The excited-state behavior in the Franck–Condon (FC) region of the d(ApG) and d(GpA) dinucleoside monophosphate (A = adenine, G = guanine) is investigated in aqueous solution by means of time-dependent Density Functional Calculations, including solvent effects by the Polarizable Continuum Model and using three different functionals, i.e. PBE0, M052X, and CAM-B3LYP. Our analysis, focussed mainly on the two stacked dimers formed by 9-methyl-adenine and 9-methyl-guanine in the same arrangement as in the B structure of DNA (AG and GA), provides absorption and ECD spectra in very good agreement with their experimental counterparts. The sequence dependence of the dinucleoside excited-state properties is fully reproduced and it is explained on the grounds of the different interactions among the nucleobases’ frontier orbitals existing in AG and GA. Our calculations predict that the 9Me-G → 9Me-A charge transfer (CT) excited state is, at least, as stable as the lowest energy bright state of the dimers and that it is more stable than the intra-strand CT state in the (9Me-A)2 stacked dimer. On the other hand, in AG and GA the 9Me-G → 9Me-A CT state is significantly coupled to the dimer bright excited states and deviations from the decay rate predicted on the ground of the Marcus Theory are possible.
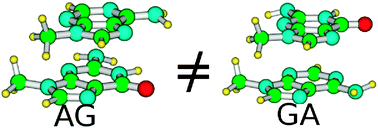
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
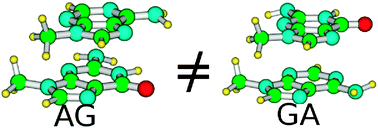

 Please wait while we load your content...
Please wait while we load your content...