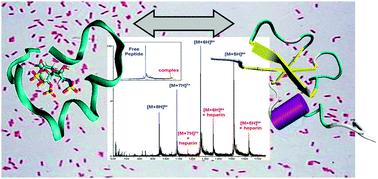
Due to the ubiquitous presence of polysaccharide moieties on bacterial surfaces, it is hypothesised that a peptide–saccharide interaction plays a key role during the recognition of invading microorganisms by β-defensins. We have employed different gas-phase methods to investigate these interactions. This manuscript describes: an MS-based titration assay measuring the gas-phase binding of ten β-defensin related peptides to a sulfated disaccharide derived from heparin (HDD); ion mobility-mass spectrometry-determined collision cross sections of 3 peptides (both free and binding HDD); and results from molecular modelling with the aim of reconciling some of our experimental observations. We observe a clear qualitative correlation between the antimicrobial activity of several β-defensins and related peptides and their gas-phase binding to a heparin-derived disaccharide (HDD). Four of the ten peptides show >100 micromolar Kd values with HDD, and no bacteriocidal activity, illustrating that HDD binding correlates with peptide antimicrobial activity. For five of the remaining six peptides, bacteriocidal activity was re-measured with HDD present. For the peptides containing intramolecular disulfide bonds in two out of five, bacteriocidal activity was reduced ∼10-fold; for the remaining three peptides, which lack intramolecular disulfide bonds, HDD addition had little effect on bacteriocidal activity. The latter results are suggested to arise from the greater degree of flexibility imparted by the removal of disulfide bonds giving the peptides the ability to envelope HDD and assume a “defensin-like” fold. Thus gas-phase analysis is put forward as a powerful tool for assessing the properties of antimicrobial peptides providing valuable insights in the mechanism of antimicrobial inhibition.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...