Synthesis and microstructure of manganese ferrite colloidal nanocrystals†
Abstract
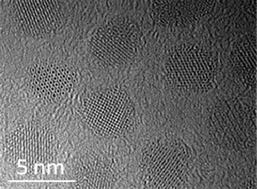
The atomic level structure of a series of monodisperse single crystalline
- This article is part of the themed collection: Molecular mechanisms of the photostability of life

 Please wait while we load your content...
Please wait while we load your content...