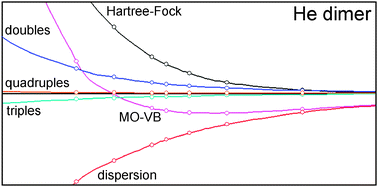
We present a short overview and two benchmark applications of the Molecular Orbital–Valence Bond (MO–VB) method, a quantum mechanical scheme developed within the framework of modern Valence Bond theory. The MO–VB has been especially designed to deal with weak intermolecular interactions, and in recent years it has been successfully applied to compute the potential energy surface of several complexes, namely He2, He–H2O, He–CH4 and Ne–CH4. In this investigation we test extensively the performance of the MO–VB on two limit systems, the He dimer and the LiH–He complex, which span three extremes in the field of van der Waals interactions: a purely dispersive system (He⋯He) and, thanks to the highly polar Li–H bond, a noble gas approaching a cation (He⋯Li+), and an anion (He⋯H−). Very accurate computations are available in the literature for He2 and LiH–He, performed either with conventional Molecular Orbital or Monte Carlo approaches, and hence these systems are the ideal candidates to establish the capabilities of the MO–VB. Based on the results of our study, we conclude that in He2 and LiH–He the MO–VB overestimates the correlation energy contribution to the interaction energy of about 5%, and hence it is a valid option for computing accurate lower bounds to the potential energy surface of these complexes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...