Phase behaviour and conductivity study on multi-component mixtures for electrodeposition in supercritical fluids
Abstract
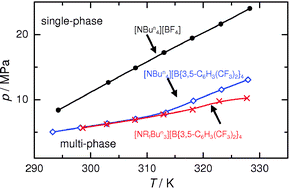
Electrochemistry in supercritical CO2 (scCO2) is difficult because the very low dielectric constant of the fluid restricts the solubility of ionic species and the conductivity of dissolved electrolytes. To overcome this problem to allow us to carry out

 Please wait while we load your content...
Please wait while we load your content...