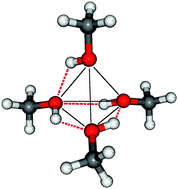
Pathways for hydrogen bond switching in a tetrameric methanol cluster
Abstract
Computational techniques (second order Møller–Plesset MP2 perturbation theory in conjunction with medium and large size basis sets) are applied to explore structural aspects of a hydrogen-bonded tetrameric cluster of

 Please wait while we load your content...
Please wait while we load your content...