Protein–DNA binding specificity: a grid-enabled computational approach applied to single and multiple proteinassemblies†
Abstract
We use a physics-based approach termed
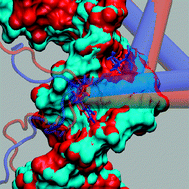
- This article is part of the themed collection: Nucleic acid simulations

 Please wait while we load your content...
Please wait while we load your content...