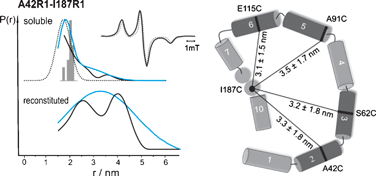
Colicin A is a water-soluble pore-forming protein that kills cells, which are not protected by an immunity protein, by inserting specific helical segments of the toxin subdomain into the cytoplasmic membrane to form voltage-dependent ion channels. This leads to depolarization of the cell membrane followed by depletion of the intracellular ATP levels and finally to cell death. The formation of the integral membrane voltage-gated ion channel is known to be accompanied by a conformational transition. Using double electron electron resonance spectroscopy inter-spin distances in doubly spin labeled colicin A mutants, with spin labels bound to positions 42/187, 62/187, 91/187 and 115/187, have been determined to serve as constraints for the modeling of the membrane bound, closed channel state of colicin A. The data reveal a quasi-circular arrangement of the eight amphipathic helices, embedded in the membrane interfacial layer close to the lipid–water interface, whereas the two hydrophobic helices are buried within the membrane.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...