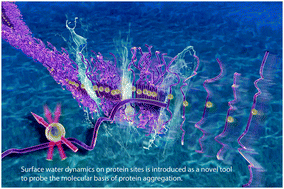
We present a generally applicable approach for monitoring protein aggregation by detecting changes in surface hydration water dynamics and the changes in solvent accessibility of specific protein sites, as protein aggregation proceeds in solution state. This is made possible through the Overhauser dynamic nuclear polarization (DNP) of water interacting with stable nitroxide spin labels tethered to specific proteins sites. This effect is highly localized due to the magnetic dipolar nature of the electron–proton spin interaction, with >80% of their interaction occurring within 5 Å between the unpaired electron of the spin label and the proton of water. We showcase our tool on the aggregation of tau proteins, whose fibrillization is linked to neurodegenerative disease pathologies known as taupathies. We demonstrate that the DNP approach to monitor local changes in hydration dynamics with residue specificity and local contrast can distinguish specific and neat protein–protein packing leading to fibers from non-specific protein agglomeration or precipitation. The ability to monitor tau assembly with local, residue-specific, resolution, under ambient conditions and in solution state will help unravel the mechanism and structural characteristics of the gradual process of tau aggregation into amyloid fibers, which remains unclear to this day.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...