Role of vibrational anharmonicity in atmospheric radical hydrogen-bonded complexes†
Abstract
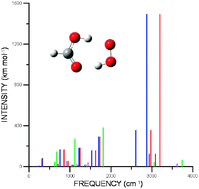
Harmonic and anharmonic vibrational frequency calculations are reported for the most stable hydrogen bonded complexes formed between the hydroperoxyl radical and formic, acetic, nitric, and sulfuric acids which are of atmospheric interest. A comparison between the calculated IR spectra of the hydrogen bonded complexes with the corresponding separate monomers is also reported with the aim to facilitate a possible experimental identification of these complexes. The calculations have been carried out using the second-order vibrational perturbative treatment implemented by Barone applied to the PES obtained with the B3LYP functional using the 6-31+G(d,p) and 6-311+G(2d,2p) basis sets. Our calculations for the separate monomers predict vibrational frequencies with quite a good agreement with the experimental values. The anharmonic contribution results in differences of around 40 cm−1 with respect to the harmonic values; although in some cases involving highly anharmonic modes, these differences can rise up to 300 and 450 cm−1.

 Please wait while we load your content...
Please wait while we load your content...