The effect of the NH2 substituent on NH3: hydrazine as an alternative for ammonia in hydrogen release in the presence of boranes and alanes†
Abstract
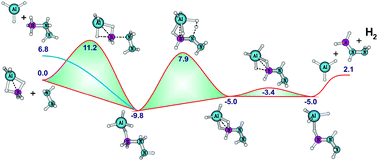
Potential energy surfaces for H2 release from hydrazine interacting with borane, alane, diborane, dialane and borane–alane were constructed from MP2/aVTZ geometries and zero point energies with single point energies at the CCSD(T)/aug-cc-pVTZ level. With one borane or alane molecule, the energy barrier for H2-loss of ∼38 or 30 kcal mol−1 does not compete with the B–N or Al–N bond cleavage (∼30 or ∼28 kcal mol−1). The second borane or alane molecule can play the role of a bifunctional catalyst. The barrier energy for H2-elimination is reduced from 38 to 23 kcal mol−1, or 30 to 20 kcal mol−1 in the presence of diborane or dialane, respectively. The mixed borane–alane dimer reduces the barrier energy for H2 release from hydrazine to ∼17 kcal mol−1. A systematic comparison with the reaction pathways from ammonia borane shows that hydrazine could be an alternative for ammonia in producing borane amine derivatives. The results show a significant effect of the NH2 substituent on the relevant thermodynamics. The B–N dative bond energy of 31 kcal mol−1 in NH2NH2BH3 is ∼5 kcal mol−1 larger than that of the parent BH3NH3. The higher thermodynamic stability could allow hydrazine–borane to be used as a material for certain energetic H2 storage applications.

 Please wait while we load your content...
Please wait while we load your content...