On the chemical potential of a component in a metastable phase—application to Li-storage in the RuO2–Li system
Abstract
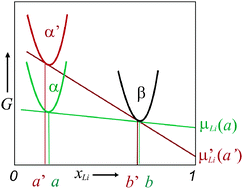
The chemical potential of a component of a binary metastable compound is considered in the single-phase and in the compositionally non-variant two-phase regime. A detailed thermodynamic analysis reveals striking differences for identical nominal compositions. Without the loss of generality the chemical potential of Li in Li containing compounds is referred to. In the single-phase regime an increased Gibbs energy of the metastable phase, compared with the stable phase, leads to an increased chemical potential of lithium, as long as we can ignore the dependence of the excess value on composition. If, in the same approximation, this metastable phase is in two-phase equilibrium with a binary phase more rich in Li, a decrease in the chemical potential and hence an inversion of the sign of the cell voltage in a Li-based battery is predicted. To be specific we consider Li-storage within RuO2 as well as in the pseudo-binary two-phase system RuO2–LiRuO2. A significantly greater cell voltage vs. Li is observed in the two-phase region if amorphous RuO2 is used instead of crystalline RuO2, in contrast to the single-phase system. A possible applicability of metastable phases for Li-based batteries is discussed.

 Please wait while we load your content...
Please wait while we load your content...