In situ investigation of molecular kinetics and particle formation of water-dispersible titania nanocrystals†
Abstract
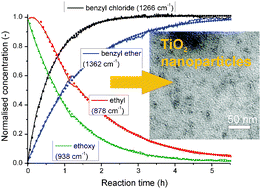
Metal oxide nanoparticles can be fabricated under high control via nonaqueous sol–gel synthesis. This route has been shown to lead to highly crystalline, uniform nanostructures, which explains the high and growing interest it is receiving. The underlying mechanisms are, however, so far only rudimentarily understood on a molecular scale. Here, we applied in situ FTIR spectroscopy and other techniques to monitor the nonaqueous synthesis of titania nanoparticles that can be easily stabilised in polar solvents and thus, possess high potential for application. A special focus is put on the kinetics of the organic condensation mechanisms enabling the reaction of the precursor to the inorganic nanoparticles. By comparing these kinetics to the process of nanoparticle formation monitored via complementary methods such as TEM and dynamic light scattering, a detailed insight into the principles and mechanisms of nanoparticle formation via the nonaqueous sol–gel synthesis is achieved.
- This article is part of the themed collection: Chemistry and physics of metal oxide nanostructures

 Please wait while we load your content...
Please wait while we load your content...