Prediction and characterization of the HMgH⋯LiX (X = H, OH, F, CCH, CN, and NC) complexes: a lithium–hydride lithium bond
Abstract
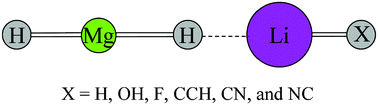
In the present paper, a new type of lithium bonding complex HMgH⋯LiX (X = H, OH, F, CCH, CN, and NC) has been predicted and characterized. Their geometries (C∞v) with all real harmonic vibrational frequencies were obtained using the second-order Møller–Plesset perturbation theory (MP2) with 6-311++G(d,p) basis set. For each HMgH⋯LiX complex, a lithium bond is formed between the negatively charged H atom of an HMgH molecule and the positively charged Li atom of an LiX molecule. Due to the formation of the complexes, the Mg–H and Li–H bonds are elongated. Interestingly, the Li–X harmonic vibrational stretching frequency is blueshifted in the HMgH⋯LiX (Y = CCH, CN, and NC) complexes and redshifted in the HMgH⋯LiX (X = H, OH, and F) complexes. The binding energy of this type of lithium bond ranges from 12.18 to 15.96 kcal mol−1, depending on the chemical environment of the lithium. The nature of lithium–hydride lithium bond has also been analyzed with natural bond orbital (NBO) and atoms in molecules (AIM).

 Please wait while we load your content...
Please wait while we load your content...