Ring-opening reaction of a trifluorinated indolylfulgide: mode-specific photochemistry after pre-excitation†
Abstract
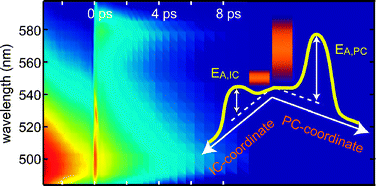
The ring-opening reaction of a trifluorinated indolylfulgide has been studied as a function of temperature and optical pre-excitation where it was found that reaction times decreased as temperature increased from 10.3 ps at 12 °C to 7.6 ps at 60 °C. Simultaneously, the quantum yields for the ring-opening reaction grew from 3.1% (12 °C) to 5.0% (60 °C). When the reaction was started from a non-equilibrium state generated by a directly preceding ring-closure process, the ring-opening reaction became faster and the quantum efficiency increased by more than a factor of three. Analysis of the experimental results points to mode-specific photochemistry in that the promoting, photochemically active modes of the photoreaction are efficiently excited by the directly preceding ring-closure reaction.

 Please wait while we load your content...
Please wait while we load your content...