Theoretical study of intermolecular interactions in meso-tetraphenylporphyrin diacid dimer (H4TPPCl2)2†
Abstract
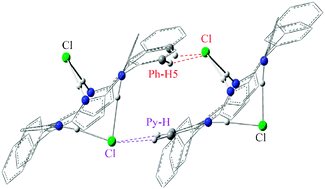
The intermolecular interactions between two meso-tetraphenylporphyrin diacid H4TPPCl2 monomers are investigated by density functional theory with the PBE1PBE functional and 6-31G* basis set. Structures of five stable isomers of (H4TPPCl2)2 are determined. It is found that the interaction (IMHB-1) of Cl with orthoH atoms in two phenyl groups of H4TPPCl2 is unique in that it is the strongest interaction between two H4TPPCl2 monomers. Natural bond orbital analysis is carried out to explain the subtle differences of hydrogen bondings in these isomers. To understand the interactions of

 Please wait while we load your content...
Please wait while we load your content...