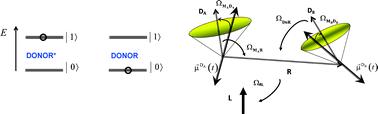
An extended Förster theory (EFT) is derived and outlined for electronic energy migration between two fluorescent molecules which are chemically identical, but photophysically non-identical. These molecules exhibit identical absorption and fluorescence spectra, while their fluorescence lifetimes differ. The latter means that the excitation probability becomes irreversible. Unlike the case of equal lifetimes, which is often referred to as, donor–donor energy migration (DDEM), the observed fluorescence relaxation is then no longer invariant to the energy migration process. To distinguish, the present case is therefore referred to as partial donor–donor energy migration (PDDEM). The EFT of PPDEM is described by a stochastic master equation (SME), which has been derived from the stochastic Liouville equation (SLE) of motion. The SME accounts for the reorienting as well as the translational motions of the interacting chromophores. Synthetic fluorescence lifetime and depolarisation data that mimics time-correlated single photon counting experiments have been generated and re-analysed. The rates of reorientation, as well as the orientational configurations of the interacting D-groups were examined. Moreover the EFT of PPDEM overcomes the classical “κ2-problem” and the frequently applied approximation of 〈κ2〉 = 2/3 in the data analyses. An outline for the analyses of fluorescence lifetime and depolarisation data is also given, which might prove applicable to structural studies of D-labelled macromolecules, e.g. proteins. The EFT presented here brings the analyses of PDDEM data to the same level of molecular detail as that used in ESR- and NMR-spectroscopy.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...