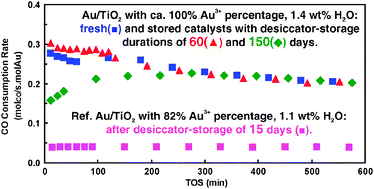
The effect of Au3+ percentage in Au/TiO2 on its storage stability at room temperature was studied by varying the drying temperature and storage duration of a deposition–precipitation prepared Au/TiO2 sample. Carefully-designed room temperature storage in a desiccator, in the dark to exclude any interference of light irradiation, was referenced to the freezing storage (255 K) in a refrigerator. The samples were characterized by well-calibrated H2-TPR, TEM and TG measurements. Reduction of Au3+ ions and agglomeration of metallic Au particles were shown to be the main reasons for the deterioration of Au/TiO2 during desiccator-storage. Correlating the percentage of Au3+ ions, determined by H2-TPR, with the storage stability of Au/TiO2 for CO oxidation at 273 K revealed that Au/TiO2 samples with higher Au3+ percentages (>90%) were much more stable during the desiccator-storage than those with higher percentages of metallic Au. Residual water in fresh Au/TiO2 before storage showed a promotional effect on gold reduction and agglomeration during storage. By maximizing the percentage of Au3+ ions and minimizing the residual water in the fresh sample, the deterioration of the Au/TiO2 catalyst was successfully avoided during desiccator-storage of up to 150 days in dark. A possible mechanism of Au/TiO2 deterioration during the desiccator-storage was also discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...