The intermolecular potentials of the O2–O2 dimer: a detailed ab initio study of the energy splittings for the three lowest multiplet states
Abstract
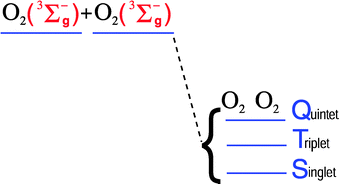
Intermolecular potentials for the three lowest multiplet states (singlet, triplet and quintet) of the O2(3Σ−g)–O2(3Σ−g) dimer have been investigated in detail by means of high level ab initio calculations. The methods used include MRCI, ACPF, CASPT2, using different active spaces and basis sets. The results for the quintet state are compared with benchmark CCSD(T) calculations. As expected, the former methods do not account accurately for dispersion interactions, although the CASPT2 method performs better than the CI based ones. On the other hand, it is shown that highly correlated methods are necessary to accurately describe the splittings among the multiplet states. We propose to obtain singlet and triplet interaction potentials by combining CCSD(T) quintet potentials and multiconfigurational singlet–quintet and triplet–quintet splittings, respectively. The calculated splittings are quite stable regarding the method employed, except for the well region of the singlet and triplet states within the rectangular configuration, which corresponds to the absolute minima of these multiplet states. Nevertheless, we have been able to assess adequate upper and lower bounds to the interaction potential for this particular region.

 Please wait while we load your content...
Please wait while we load your content...