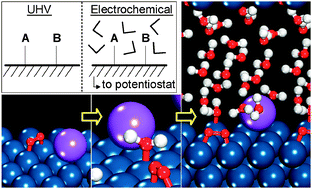
Influences of coadsorbed sodium and water, aqueous solvent, and electrode potential on the kinetics of O2 dissociation over Pt(111) are systematically investigated using density functional theory models of vacuum and electrochemical interfaces. Na coadsorption alters the electronic states of Pt to stabilize the reactant (O2*), transition, and product (2O*) states by facilitating electron donation to oxygen, causing a more exothermic reaction energy (−0.84 eV for Na and O2, −0.81 eV for isolated O2) and a decrease in dissociation barrier (0.39 eV for Na and O2, 0.57 eV for isolated O2). Solvation decreases the reaction energy (−0.67 eV) due to enhanced hydrogen bond stabilization of O2* compared to 2O*. The influence of Na is less pronounced at the solvated interface (barrier decreases by only 0.11 eV) because H2O screens Na charge-donation. In the electrochemical model system, the dissociation energy becomes more exothermic and the barrier decreases toward more positive potentials. Potential-dependent behavior results from changes in interfacial dipole moment and polarizability between O2*, the dissociation transition state, and 2O*; each are influenced by changes in adsorption and hydrogen bonding. Coadsorption of Na in the solvated system dampens the dipole moment change between O2* and 2O* and significantly increases the polarizability at the dissociation transition state and for 2O*; the combination causes little change in the reaction energy but reduces the activation barrier by 0.08 eV at 0 V versusNHE. The potential-dependent behavior contrasts that determined at a constant surface charge or from an applied electric field, illustrating the importance of considering the electrochemical potential at the fully-solvated interface in determining reaction energetics, even for non-redox reactions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...