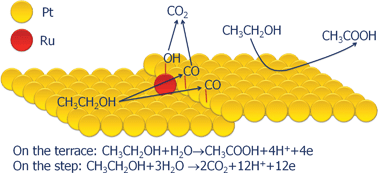
Oxidation of ethanol on ruthenium-modified Pt(775) and Pt(332) stepped electrodes has been studied using electrochemical and FTIR techniques. It has been found that the oxidation of ethanol on these electrodes takes place preferentially on the step sites yielding CO2 as the major final product. The cleavage of the C–C bond, which is the required step to yield CO2, occurs only on this type of site. The presence of low ruthenium coverages on the step sites promotes the complete oxidation of ethanol since it facilitates the oxidation of CO formed on the step from the cleavage of the C–C bond. However, high ruthenium coverages have an important inhibiting effect since the adatoms block the step sites, which are required for the cleavage of the C–C bond. Under these conditions, the oxidation current diminishes and the major product in the oxidation process is acetic acid, which is the product formed preferentially on the (111) terrace sites.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...