The structural and electronic properties of the excited electronic states of AgX2 (X = F, Cl, Br, and I), have been calculated, taking electron correlation and spin–orbit coupling into account and employing improved relativistic-effective-core potentials for silver and the halogen atoms. The relative ordering of the excited states of these molecules has been discussed via molecular-orbital arguments. The spin–orbit splittings of three degenerate electronic states (2Πg, 2Πu, and 2Δg) have been calculated and the spin–orbit induced inter-state (Σ − Π) coupling has been discussed. The composition of the spin–orbit eigenstates is analyzed in terms of scalar-relativistic electronic states. Finally, a theoretical prediction of the photodetachment bands of the title molecules has been accomplished.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
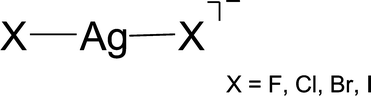

 Please wait while we load your content...
Please wait while we load your content...