Aluminium-doped LiFePO4 single crystals
Part II.† Ionic conductivity, diffusivity and defect model
Abstract
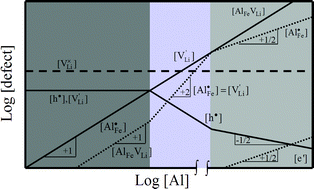
We report on lithium ion conductivity and diffusivity along major crystallographic directions of Al-doped LiFePO4 single crystals.

 Please wait while we load your content...
Please wait while we load your content...