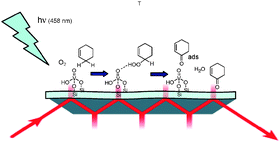
Vanadia was incorporated in the 3-D mesoporous material TUD-1 with a loading of 2% w/w vanadia. The performance in the selective photo-oxidation of liquid cyclohexene was investigated using ATR-FT-IR spectroscopy. Under continuous illumination at 458 nm a significant amount of product, i.e.cyclohexenone, was identified. This demonstrates for the first time that hydroxylated vanadia centers in mesoporous materials can be activated by visible light to induce oxidation reactions. Using the rapid scan method, a strong perturbation of the vanadyl environment could be observed in the selective oxidation process induced by a 458 nm laser pulse of 480 ms duration. This is proposed to be caused by interaction of the catalytic centre with a cyclohexenyl hydroperoxide intermediate. The restoration of the vanadyl environment could be kinetically correlated to the rate of formation of cyclohexenone, and is explained by molecular rearrangement and dissociation of the peroxide to ketone and water. The ketone diffuses away from the active center and ATR infrared probing zone, resulting in a decreasing ketone signal on the tens of seconds time-scale after initiation of the photoreaction. This study demonstrates the high potential of time resolved ATR FT-IR spectroscopy for mechanistic studies of liquid phase reactions by not only monitoring intermediates and products, but also by correlating the temporal behavior of these species to molecular changes of the vanadyl catalytic site.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...