The progression of strong and weak hydrogen bonds in a series of ethylenediammonium dithiocyanate derivatives—a new bonding protocol for macromolecules?†
Abstract
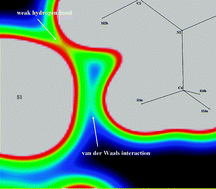
The experimental charge densities for a series of sym-N-methyl-substituted ethylenediammonium dithiocyanate salts have been investigated based on low-temperature and

 Please wait while we load your content...
Please wait while we load your content...