Through-space interactions between parallel-offset arenes at the van der Waals distance: 1,8-diarylbiphenylene syntheses, structure and QM computations†
Abstract
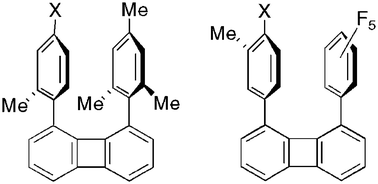
A model for studying polar–π interactions between
- This article is part of the themed collection: Stacking interactions

 Please wait while we load your content...
Please wait while we load your content...