Experimental and theoretical determination of the accurate interaction energies in benzene–halomethane: the unique nature of the activated CH/π interaction of haloalkanes†
Abstract
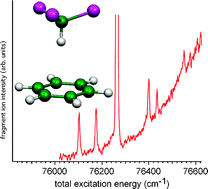
The CH/π interaction energies between
- This article is part of the themed collection: Stacking interactions

 Please wait while we load your content...
Please wait while we load your content...