Cluster-assembled compounds comprising an all-metal subunit Li3Al4− †
Abstract
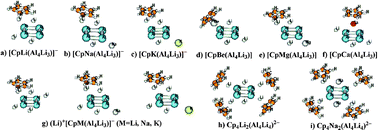
The recent, experimentally-discovered, all-metal antiaromatic Li3Al4− has attracted great interest and extensive investigations due to its unique chemical bonds and exotic properties. Although a very recent theoretical study demonstrated that the all-metal species Li3Al4− can be effectively stabilized by complexation with 3d transition metals, unfortunately such stabilization is at the expense of losing antiaromaticity (rectangular Al4) to become aromatic (square Al4). Here, we predict theoretically a series of cluster-assembled compounds [DM(Li3Al4)]q− (D = Li3Al4−, Cp−; M = Li, Na, K, Be, Mg, Ca). The assembled species are ground states containing the all-metal antiaromatic Li3Al4− subunits. Many fusion isomers are energetically lower than the homo-decked cluster-assembled compounds, thus, the homo-decked assembly species [M(Li3Al4)2]q− are less likely due to their thermodynamic instability. In addition, the well-retained all-metal antiaromaticity is mainly ascribed to the ionic electrostatic interactions and the protections of rigid organic aromatic Cp-deck avoiding the fusion of Li3Al4−. Our results represent the first example that the all-metal antiaromaticity is well retained in assembled compounds as that in the free Li3Al4−cluster. Sufficiently large interaction energies make the realization of all-metal antiaromatic Li3Al4−-incorporated compounds very promising.

 Please wait while we load your content...
Please wait while we load your content...