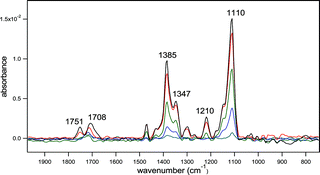
The ozonolysis of an approximately one monolayer film of 1-oleoyl-2-palmitoyl-sn-glycero-3-phosphocholine (OPPC) on NaCl was followed in real time using diffuse reflection infrared Fourier transform spectrometry (DRIFTS) at 23 °C. Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry and Auger electron spectroscopy were used to confirm the identification of the products. Ozone concentrations ranged from 1.7 × 1012 to 7.0 × 1013 molecules cm–3 (70 ppb to 2.8 ppm). Upon exposure to O3, there was a loss of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C accompanied by the formation of a strong band at ∼1110 cm–1 due to the formation of a stable secondary ozonide (1,2,4-trioxolane, SOZ). The yield of the SOZ was smaller when the reaction was carried out in the presence of water vapor at concentrations corresponding to relative humidities between 2 and 25%. The dependencies of the rate of SOZ formation on the concentrations of ozone and water vapor are consistent with the initial formation of a primary ozonide (1,2,3-trioxolane, POZ) that can react with O3 or H2O in competition with its thermal decomposition to a Criegee intermediate and aldehyde. Estimates were obtained for the rate constants for the POZ thermal decomposition and for its reactions with O3 and H2O, as well as for the initial reaction of O3 with OPPC. The SOZ decomposed upon photolysis in the actinic region generating aldehydes, carboxylic acids and anhydrides. These studies show that the primary ozonide has a sufficiently long lifetime when formed on a solid substrate that direct reactions with O3 and H2O can compete with its thermal decomposition. In dry polluted atmospheres, ozone–alkene reactions may lead in part to the formation of stable secondary ozonides whose chemistry, photochemistry and toxicity should be taken into account in models of such regions.
C accompanied by the formation of a strong band at ∼1110 cm–1 due to the formation of a stable secondary ozonide (1,2,4-trioxolane, SOZ). The yield of the SOZ was smaller when the reaction was carried out in the presence of water vapor at concentrations corresponding to relative humidities between 2 and 25%. The dependencies of the rate of SOZ formation on the concentrations of ozone and water vapor are consistent with the initial formation of a primary ozonide (1,2,3-trioxolane, POZ) that can react with O3 or H2O in competition with its thermal decomposition to a Criegee intermediate and aldehyde. Estimates were obtained for the rate constants for the POZ thermal decomposition and for its reactions with O3 and H2O, as well as for the initial reaction of O3 with OPPC. The SOZ decomposed upon photolysis in the actinic region generating aldehydes, carboxylic acids and anhydrides. These studies show that the primary ozonide has a sufficiently long lifetime when formed on a solid substrate that direct reactions with O3 and H2O can compete with its thermal decomposition. In dry polluted atmospheres, ozone–alkene reactions may lead in part to the formation of stable secondary ozonides whose chemistry, photochemistry and toxicity should be taken into account in models of such regions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C accompanied by the formation of a strong band at ∼1110 cm–1 due to the formation of a stable secondary
C accompanied by the formation of a strong band at ∼1110 cm–1 due to the formation of a stable secondary 

 Please wait while we load your content...
Please wait while we load your content...