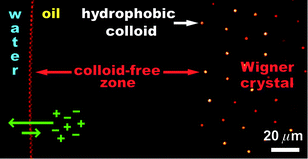
We present a combined experimental and theoretical investigation of the surprisingly strong electrostatic effects that can occur in mixtures of low- and high-polar liquids (e.g. oil–water emulsions), here in the presence of colloidal particles. For our experiments, we used confocal microscopy imaging, supplemented with electrophoresis and conductivity measurements. Theoretically, we studied our systems by means of a modified Poisson–Boltzmann theory, which takes into account image charge effects and the electrostatic self-energies of the micro-ions in the different dielectric media. Our results show that the unequal partitioning of micro-ions between the two liquid phases is the common driving force behind most of the observed electrostatic effects. The structural signatures of these effects typically develop on a time scale of hours to days and are qualitatively well-described by our theory. We demonstrate how the partitioning process and its associated phenomena can be controlled by shifting the balance of the interlocked ionic dissociation and partitioning equilibria. Moreover, we present strong experimental proof that the two-dimensional colloidal crystals at the oil–water interface are due to long-ranged Coulombic repulsion through the oil phase. The acquired insight in the role of electrostatics in oil–water emulsions is important for understanding the interactions in particle-stabilized (‘Pickering’) and charge-stabilized emulsions, emulsion production, encapsulation and self-assembly.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...