A mechanism for the decomposition of dinitropropyl compounds
Abstract
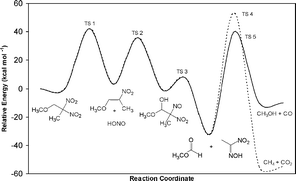
A decomposition mechanism is proposed for 2,2-dinitro-1-methoxypropane, a compound whose structure resembles the nitroplasticizer (NP) component of plastic-bonded explosive PBX 9501. A library of key reactions is presented and is based on the results of NP aging studies and existing decomposition mechanisms for similar nitro compounds. Density functional electronic structure calculations on these reactions were used to develop a decomposition mechanism at lower temperatures, which begins with HONO elimination and leads to intermediates that can produce CO, CO2, NO, and N2O gases. These gases were observed in low temperature (48 to 64 °C) aging studies of NP. A high temperature mechanism involving NO2 scission is compared to a thermal decomposition mechanism determined by simultaneous thermogravimetric modulated beam mass spectrometry. The calculated energy barriers for HONO elimination and NO2 scission in the gas phase are reported and compared to experimental results.

 Please wait while we load your content...
Please wait while we load your content...