Nernst–Planck analysis of propagating reaction-diffusion fronts in the aqueous iodate–arsenous acid system
Abstract
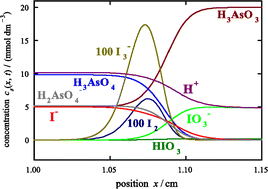
Propagating fronts can be generated in solution by combining diffusion and chemical reactions with an autocatalytic feedback mechanism. Front propagation is usually analyzed in terms of the rate equations for the chemical reactions and Fick’s laws of molecular diffusion. In practice, however, reaction-diffusion fronts are known mainly for aqueous electrolyte solutions. A more accurate description of front propagation in these systems is developed by using Nernst–Planck (NP) transport equations. This treatment includes diffusion fluxes driven by the concentration gradients and, for the ionic species, the migration fluxes driven by the electric field which is generated internally by the diffusion of ions of different mobility. NP equations are used to describe propagating fronts for the

 Please wait while we load your content...
Please wait while we load your content...