Electrocatalytic oxidation of ammonia on Pt(111) and Pt(100) surfaces
Abstract
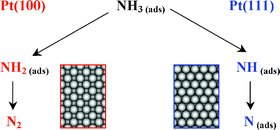
The electrocatalytic oxidation of ammonia on Pt(111) and Pt(100) has been studied using voltammetry, chronoamperometry, and in situ infrared spectroscopy. The oxidative adsorption of ammonia results in the formation of NHx (x = 0–2) adsorbates. On Pt(111), ammonia oxidation occurs in the double-layer region and results in the formation of NH and, possibly, N adsorbates. The experimental current transients show a hyperbolic decay (t−1), which indicates strong lateral (repulsive) interactions between the (reacting) species. On Pt(100), the NH2 adsorbed species is the stable intermediate of ammonia oxidation. Stabilization of the NH and NH2 fragments on Pt(111) and Pt(100), respectively, is in an interesting agreement with recent theoretical predictions. The Pt(111) surface shows extremely low activity in ammonia oxidation to dinitrogen, thus indicating that neither NH nor N (strongly) adsorbed species are active in dinitrogen production. Neither nitrous oxide nor nitric oxide is the product of ammonia oxidation on Pt(111) at potentials up to 0.9 V, as deduced from the in situ infrared spectroscopy measurements. The Pt(100) surface is highly active in dinitrogen production. This process is characterized by a Tafel slope of 30 mV decade−1, which is explained by a rate-determining dimerization of NH2 fragments followed by a fast decay of the resulting surface-bound hydrazine to dinitrogen. Therefore, the high activity of the Pt(100) surface for ammonia oxidation to dinitrogen is likely to be related to its ability to stabilize the NH2 adsorbate.

 Please wait while we load your content...
Please wait while we load your content...