Tropospheric multiphase chemistry of 2,5- and 2,6-dimethylphenols: determination of the mass accommodation coefficients and the Henry’s law constants
Abstract
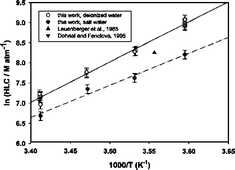
The uptake of 2,5-dimethylphenol and 2,6-dimethylphenol on aqueous surfaces was measured between 279 and 293 K, using the wetted-wall flow tube technique coupled with UV absorption spectroscopic detection. For both compounds, the uptake coefficients γ were found to be independent of the KOH scavenger concentration in the range of 0.01 to 1 M (pH > pKa) and of the liquid–gas contact times. In addition, the uptake coefficients and the derived mass accommodation coefficients α show a negative temperature dependence in the investigated temperature range. The mass accommodation coefficients decrease from 1.1 × 10−3 to 1.1 × 10−4, and from 5.4 × 10−4 to 6.4 × 10−5 for 2,5-dimethylphenol and 2,6-dimethylphenol, respectively. These results are used to discuss the incorporation of these species into the liquid using the nucleation theory. Henry’s law constants (HLC) of both compounds were directly measured using a dynamic equilibrium system based on the water/air equilibrium at the interface within the length of a microporous tube. The measurements were conducted over the range 278–293 K in both deionized water and 35 g L−1 solution of NaCl. At 293 K and in pure water, HLC were found to be equal to (in units of M atm−1): 2,5-dimethylphenol, HLC = (1270 ± 240); 2,6-dimethylphenol, HLC = (250 ± 80). All of the values for HLC in 35 g L−1 salt solution were 5–55% lower than the corresponding values in deionized water, depending on the compound and the temperature. These data (mass accommodation coefficients and Henry’s law constants) were then used to estimate the partitioning of these phenolic compounds between gaseous and aqueous phases and the corresponding atmospheric lifetimes under clear sky (τgas) and cloudy conditions (τmultiphase) have then been derived. The calculated multiphase lifetimes (in units of hours) are lower than those in gas phase at a cumulus temperature of 283 K (in parentheses): 2,5-dimethylphenol, 2.2 (3.5); 2,6-dimethylphenol, 3.8 (4.2).

 Please wait while we load your content...
Please wait while we load your content...