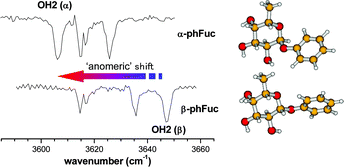
The conformation and structure of phenyl-α-L-fucopyranoside (α-PhFuc), phenyl-β-L-fucopyranoside (β-PhFuc) and their singly hydrated complexes (α,β-PhFuc·H2O) isolated in a molecular beam, have been investigated by means of resonant two photon ionization (R2PI) spectroscopy and ultraviolet and infrared ion-dip spectroscopy. Conformational and structural assignments have been based on comparisons between their experimental and computed near IR spectra, calculated using density functional theory (DFT) and their relative energies, determined from ab initio (MP2) calculations. The near IR spectra of ‘free’ and hydrated α- and β-PhFuc, and many other mono- and di-saccharides, provide extremely sensitive probes of hydrogen-bonded interactions which can be finely tuned by small (or large) changes in the molecular conformation. They provide characteristic ‘signatures’ which reflect anomeric, or axial vs. equatorial differences, both revealed through comparisons between α/β-PhFuc and α/β-PhXyl; or similarities, revealed through comparisons between fucose (6-deoxy galactose) and galactose; or binding motifs, for example, ‘insertion’
vs. ‘addition’ structures in hydrated complexes. At the monosaccharide level (the first step in the carbohydrate hierarchy), these trends appear to be general. In contrast to the monohydrates of galactose (β-PhGal) and glucose (β-PhGlc), the conformations of α- and β-PhFuc are unaffected by the binding of a single water molecule though changes in the R2PI spectra of multiply hydrated α-PhFucW(n) however, may reflect a conformational transformation when n
≥ 3.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...